

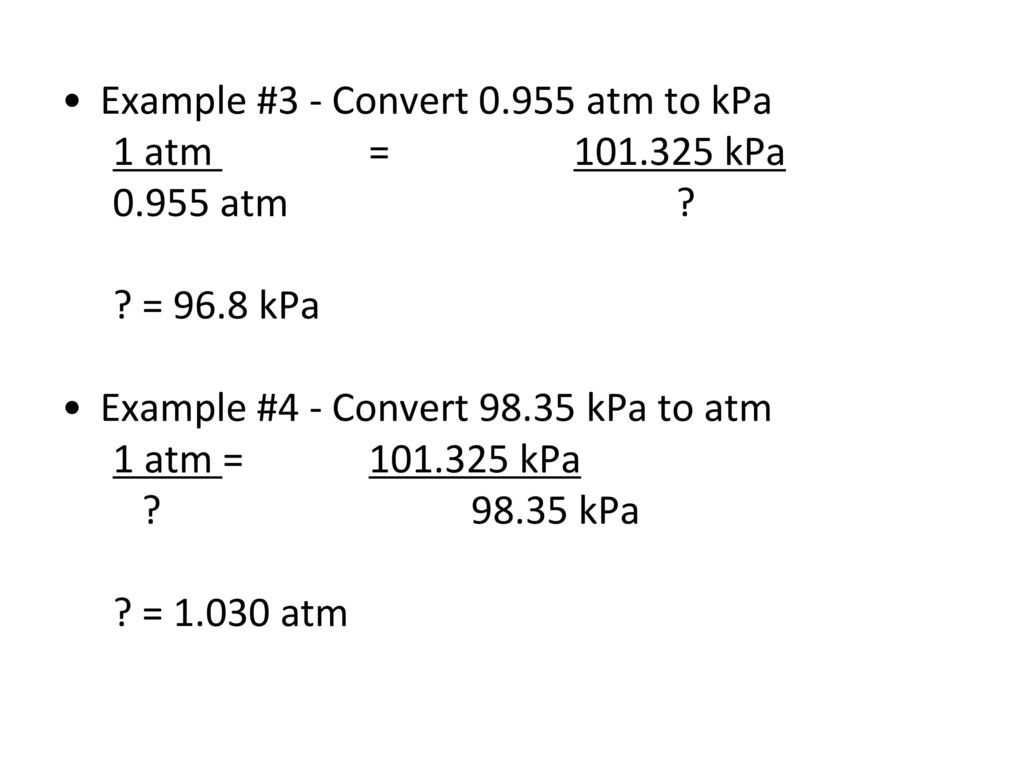
And we also know that one mmHg is also equal to 1 torr, which is 1 / 760 of atmospheric pressure (atm) that is 1 atm = 760 mmHg.
#350 mmhg to atm how to#
How to calculate mmHg?Īs discussed earlier we know that one millimetre of mercury is the pressure exerted by a 1mm vertical column of mercury at 0 degree Celsius. In addition, they displayed the pressure alteration among two fluids as a vertical variance between the mercury levels of two connected reservoirs. But, they are no longer in usage due to the toxicity of mercury.įurthermore, the sensitivity of mercury columns to temperature and local gravity and the better convenience of other instrumentation also reduce their use. Also, mercury manometer is the first and most accurate pressure gauge. Moreover, these two units are not equal and the relative difference (less than 0.000015 per cent) is minor for most practical use. In addition, one millimetre of mercury is approximately 1 Torr which is 1 / 760 of standard atmospheric pressure (101325 / 760 ≈ 133.322368421053 Pascals).
In this the mm represents millimetre and Hg is the chemical name of mercury or the symbol for mercury.įurthermore, the millimetre of mercury is still routinely used in medicine, aviation, meteorology, and many other scientific fields. Moreover, we denote it with mmHg or mm Hg. Also, it is currently defined as exactly 133.3223 Pascals. Besides, one can describe it as the extra pressure whose production takes place by a column of mercury one millimetre high. It is a unit of measure that uses the manometric unit to tell the pressure of the objects. Besides, in this topic, we will discuss what is a millimetre of mercury and how to calculate mmHg? Millimetre of mercury is the short form for a millimetre of mercury.Īlso, it is a measuring unit that measures the pressure in the manometric unit. I could have done this since I know the volume of A (which is V 1) is 4.4 times the volume of B (which is V 2).MmHg is certainly a very important calculation in Physics. fig.)ħ) Comment: I could have assigned an arbitrary volume of 1 to V 2, making the value for V 1 be 4.4. The problem specifies P and T are also constant.ĥ) Since there is a VM/m for nitrogen and a VM/m for argon, we have this:Ĭontainer A (nitrogen): V 1 = 4.4V 2, M 1 = 28.0 g/molĬontainer B (argon): V 2 = V 2, M 2 = 40.0 g/mol N = m/M, where M is the molar mass of the gas and m is the mass of the gasĢ) Substituting one into the other, we have this:ģ) Some factors are constant, some are variable: What is the mass of the Ar (in g) within container B?ġ) For this problem, there are two equations of interest: (22.4 L / 1.00 mol) = (x / 1.27 mol) 1.50 − 0.046117 = 1.45 g H 2O liquid (to three sig figs)īonus Problem #2: Container A holds N 2 gas with a mass of 56.2 g and is 4.4 times the volume of container B which holds argon (Ar) gas at the exact same temperature and pressure. Problem #16: What volume will 1.27 moles of helium gas occupy at STP? Problem #15: How many moles of a gas would be present in a gas trapped within a 37.0 liter vessel at 80.00 ☌ at a pressure of 2.50 atm? Problem #14: How many moles of gas would be present in a gas trapped within a 100.0 mL vessel at 25.0 ☌ at a pressure of 2.50 atmospheres? Note the conversion from mmHg to atm in the denominator. Problem #13: Calculate the volume 3.00 moles of a gas will occupy at 24.0 ☌ and 762.4 mm Hg. Multiply the answer (which is in atm) by 760.0 mmHg atm¯ 1 to get mmHg If we used g, the mol unit in R would not cancel and we need to have it cancel because we require atm (and only atm) to be in the answer. This is done in order to convert grams to moles, because the value for R contains mol as the unit for amount of substance. Please note the division of 1.09 by 2.02. What is the pressure in this container in mmHg? Problem #12: 1.09 g of H 2 is contained in a 2.00 L container at 20.0 ☌. If we used mmHg, the pressure units would not cancel and we need to have them cancel because we require mol (and only mol) to be in the answer. This is done in order to convert the pressure from mmHg to atm, because the value for R contains atm as the pressure unit. Problem #11: How many moles of gas are contained in 890.0 mL at 21.0 ☌ and 750.0 mm Hg pressure?
ChemTeam: Ideal Gas Law: Problems #11 - 25


 0 kommentar(er)
0 kommentar(er)
